Introduction

The Notice of Special Interest (NOSI) NOT-OD-24-001: Administrative Supplements to Recognize Excellence in Diversity, Equity, Inclusion, and Accessibility (DEIA) Mentorship recognizes the crucial role great mentors play in developing future leaders in the scientific research enterprise. Multiple NIH Institutes and Centers (ICs) are inviting applications for administrative supplements to existing NIH awards to support scientists who are outstanding mentors and who have demonstrated compelling commitments and contributions to enhancing DEIA in the biomedical and behavioral sciences.
Supplements are available for various grant types, including career development, training, cooperative, and Research Project Grants (R01). They will provide up to $250,000 in direct costs, not to exceed the direct costs of the parent award.
Applications closed on February 16, 2024.
Past Webinar
Administrative Supplements to Recognize Excellence in DEIA Mentorship Webinar
The webinar provided prospective applicants the opportunity to understand and ask questions on the scientific scope of this NOFO and technical details for applying.
Please refer to the following resources for additional assistance:
- How to submit an administrative supplement.
- Multiple Principal Investigators Frequently Asked Questions.
Please consult with the Program Officer of the parent award and the appropriate institute/center (IC) scientific contact.
Eligibility
The following criteria must be met to be eligible for the program:
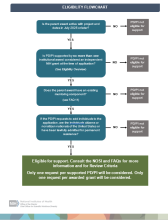
- Individual PD/PIs must be supported by no more than one institutional award considered an independent NIH grant at the time of application. Support from awards on the “smaller grants” list do not count toward the independent NIH grant award.
- Only one request per supported PD/PI will be considered. Only one request per awarded grant will be considered.
- Individuals to be added and supported with funds from this administrative supplement must be citizens or noncitizen nationals of the United States or have been lawfully admitted for permanent residence.
- Active awards with project end dates in July 2025 or later are eligible. The award may not be in a terminal no-cost extension or going into a no-cost extension in FY2024.
Eligibility FAQ
A PD/PI on a R01 and a U54 grant is ineligible for support from this administrative supplement. Support from awards on the “smaller grants” list do not count toward the independent NIH grant award (https://grants.nih.gov/policy/early-investigators/list-smaller-grants.htm). This list can help you determine eligibility. For example, if an individual is a PD/PI on two awards on that list, then they may be eligible for support from this administrative supplement. Support from grants not on that list would count toward the independent NIH grant award count, and more than one would render the PI ineligible for support.
The list of “smaller grants” determines whether the parent award counts toward the number of independent NIH grant award and not ESI status.
The table below provides additional examples to help you determine eligibility. This applies if you are named as a PD/PI on a single- or multi-PI grant.
| At the time of applying, I am PI on these grants | Are these listed as “smaller grants?” | Eligible for support from this administrative supplement? |
| Two R01s | R01: not on list and not considered a small grant | Not eligible for support. Both are counted toward independent research award. |
| U54, R21, R01 | U54: not on list R21: yes, listed and considered a smaller grant R01: not on list | Not eligible for support. Both U54 and R01 count toward independent research award. |
| R03, R01 | R03: yes, listed R01: not on list | Yes, eligible for support. Only R01 counts toward independent research award. |
| T32, R01 | T32: yes, listed R01: not on list | Yes, eligible for support. Only R01 counts toward independent research award. |
| K24, P20 | K24: yes, listed P20: not on list | Yes, eligible for support. Only P20 counts toward independent research award. |
| P50, R01 | P50: not on list R01: not on list | Not eligible for support. Both P50 and R01 count toward independent research award. |
| R25, T32 | R25: yes, listed T32: yes, listed | Yes, eligible for support. Neither R25 nor T32 count toward independent research award count. |
| I am named as multi-PI on R01 and multi-PI on U01 | R01: not on list U01: not on list | Not eligible for support. Both R01 and U01 count toward independent research award. |
Individuals named as the PD/PI are eligible regardless of being named the contact PI. Only one request per supported PD/PI will be considered. Only one request per awarded grant will be considered.
Co-investigators are not eligible individuals to apply for this administrative supplement.
No. To be eligible for support, the parent award must be active, and the research proposed in the supplement must be accomplished within the competitive segment. Active awards with project end dates in July 2025 or later are eligible. The award may not be in a terminal no-cost extension or going into a no-cost extension in FY2024.
No, you are not eligible because you are named as PD/PI on two R01s.
The “independent NIH grant award” refers only to grants from the National Institutes of Health (NIH).
The leadership at your institution (e.g., deans, provosts, etc.) reviews the criteria below and makes the determination whether your institution qualifies as an HRI or LRI. To qualify as an LRI, institutions must meet all of the following criteria:
- have received less than $50 million average in annual NIH funds within the three years prior to the time of application, and
- offer doctorate degrees in the health professions or in a health-related science, and
- have a documented historical and current mission or documented historical commitment to educating underrepresented students, and
- if institutions provide clinical health care services, those services must be provided to medically underserved communities.
These criteria are similar to the Research Centers in Minority Institutions criteria, an independent program.
HRIs are institutions that do not meet the LRI criteria.
Please work with the leadership at your institution to determine your institution’s status. They are the ones to sign the “Institutional Commitment Letter” demonstrating that your institution meets the limited-resourced institution criteria.
Individuals to be added and supported with funds from this administrative supplement must be citizens or noncitizen nationals of the United States or have been lawfully admitted for permanent residence. For the definition of citizens and noncitizen nationals, please visit:
https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title8-section1401&num=0&edition=prelim
https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title8-section1408&num=0&edition=prelim
We recognize that most K grants, especially mentored Ks, are for receiving mentorship. As such, most will not be eligible. However, if the parent award has a component for the PD/PI to provide mentorship, it may be eligible.
To learn whether an administrative supplement application can be submitted to the parent award, please review the list of participating NIH institutes and centers (listed under “issued by”). If the administering institute/center is listed, then the parent award potentially qualifies as eligible. If not, then no supplement application to the parent award can be submitted.
Please visit the NIH UNITE website. UNITE is facilitating research to identify opportunities, make recommendations, and develop and implement strategies to increase inclusivity and diversity in science.
https://www.nih.gov/ending-structural-racism/unite
Find out how UNITE is helping NIH provide research funding to drive creativity and innovation. Go to Funding Opportunities under UNITE highlights or go directly to https://extramural-diversity.nih.gov/guidedata/data.
Scope
This NOSI encourages PD/PIs to promote DEIA and mentorship and focuses on allowing mentors who have dedicated their time and effort and made significant contributions, based on evaluation data and publications, to DEIA to apply for an administrative supplement to further their personal research and/or mentoring activities. Funds will be provided to perform additional activities within the scope of the parent grant. Funds may also be provided to extend DEIA activities. Funds may also be used for additional trainee slots on an NRSA award; however, applicants must contact IC staff on the parent award. Activities must be focused within the United States and territories.
Scope FAQ
The parent award must have an existing mentoring component. Some examples are presented in the notice and include but are not limited to:
- a Diversity Supplement associated within the current competitive segment of the parent award
- a Research Education Program that describes mentored research experiences
- a Plan to Provide Mentoring
- a Plan to Enhance Diverse Perspectives
- a discrete objective related to mentoring (examples include but are not limited to a description of mentoring others in a specific aim, a section in the research strategy, or a section in the research training program plan)
Yes, having current and/or past diversity supplements on the parent award can fulfill the mentoring requirement as long as the PD/PI can show a clear commitment to their trainees’ scientific and professional development and career progress in the biomedical research enterprise.
Note: The diversity supplement must be associated with the current competitive segment of the parent award in order to fulfill the mentoring requirement.
Mentoring can be defined as “a professional, working alliance in which individuals work together over time to support the personal and professional growth, development, and success of the relational partners through the provision of career and psychosocial support” (NASEM, 2019). Mentorship provides psychosocial and career support, self-efficacy, and science identity; it is also culturally responsive (NASEM, 2019).
Mentoring activities may include providing not only technical expertise, but advice, insight, and professional career skills to college students, graduate students, post-doctorates, and/or early-career faculty as well as throughout the career trajectory. Mentorship can take the form of a dyadic relationship, one mentor working with one mentee, or a variety of configurations, including small groups, and may include any situation where “mentors and mentees...”[are]“...providers and recipients of unique information and access to resources” (NASEM, 2019). Successful mentees have the “ability to define their career goals, identify the skills they need to achieve those goals, and take the necessary steps to make progress toward those goals” (NASEM, 2019).
Examples of DEIA excellence:
- Enhancing training-based, mentoring, networking, cohort-building, career development, or psychosocial intervention to increase persistence of individuals from diverse backgrounds, including those from groups underrepresented in the biomedical research enterprise (see, Notice of NIH's Interest in Diversity, NOT-OD-20-031)
- Demonstrating overall strong commitment and contributions to enhancing DEIA in the biomedical sciences
- Fostering a diverse and inclusive research workforce and research environment for trainees from all backgrounds
- Assuming leadership roles in DEIA activities
- Engaging in DEIA service-oriented efforts beyond the needs of their own research programs
- Demonstrating positive outcomes and meaningful impact of DEIA activities
The parent award must have an existing mentoring component, not simply name a postdoc to be supported. See the NOSI NOT-OD-24-001 and FAQs 11 and 13 for information on mentoring.
No, applications with a “to-be-determined trainee slot” are not allowed. They must include each trainee’s name.
If an application asks to support a trainee, it must include the trainee’s biosketch. The trainee will include in their Personal Statement information about their motivation to enter and remain in biomedical and behavioral research and their short- and long-term career goals. There are no review criteria to assess the trainee; rather they assess the PD/PI. Since the supplement seeks to recognize outstanding mentorship, the PD/PI’s commitment to their trainee’s success and to diversity, equity, inclusion, and accessibility will be assessed.
If an application proposes to develop curricula or training activities for a cohort, then individual trainee biosketches are not required.
Yes, the application can describe mentoring of more than one trainee. We expect that the PD/PI will be able to show a record of many trainee successes coming from their lab. As this award will fund up to $250,000 in direct costs (not to exceed the current year costs of the parent grant), the PD/PI should have sufficient funds to support more than one trainee.
No, funds are available to support trainees from diverse backgrounds, including those from groups underrepresented in the biomedical research enterprise as described in the NIH Notice of Interest in Diversity.
Yes, mentoring of individuals at later career stages is allowed if well justified. See NOSI NOT-OD-24-001 “Program Description and Requirements.”
Yes, the administrative funds can be used to support (within scope) a trainee who is already supported on the grant. Trainees, previously enrolled in the T32/T35, R25 are eligible to be re-appointed as trainees under this program if within scope of the parent award. The trainee must be at the training level approved for the grant (i.e., predoctoral, postdoctoral, or short-term).
However, if proposing an increase in the number of training positions, the need for the extra positions must be justified and supported by a good record of filling previous slots and evidence of an adequate trainee pool.
This NOSI encourages PD/PIs to promote DEIA and mentorship broadly and as such differs from a Diversity Supplement (PA-23-189). The latter is designed to support research experiences for individuals from diverse backgrounds, including those from underrepresented groups, through the career continuum from high school to junior faculty levels. In contrast, this NOSI focuses on allowing mentors who have dedicated their time and effort and made significant contributions, based on evaluation data and publications, to DEIA to apply for an administrative supplement to further their personal research and/or mentoring activities. As such, the activities proposed in the application should be distinguishable from a standard diversity supplement application.
The following types of applications are not responsive to this NOSI and will not be reviewed:
- Applications only requesting salary support or funds for research equipment/supplies for investigators who are developing their research career akin to the approach of a Diversity Supplement
- Applications to supplement a parent award lacking a mentoring component
- Applications without an explicit mentoring objective in the parent award
- Applications lacking the following information in the list of trainees:
- each trainee’s name
- start/end dates of training
- summary of support during training (e.g., grant support and technical skills development, opportunities for networking, leadership)
- terminal degree received and year
- position while a trainee (e.g., graduate student, post-doctorate, etc.)
- current position
- PD/PI’s outreach efforts to enhance DEIA
- description of outcomes
- Applications lacking a DEIA Statement
Please consult the program officer on the parent award. Additionally, you may include the appropriate point of contact for the NIH institute or center administering your award. Those individuals are listed above.
Budget
Application budgets should not exceed $250,000 in direct costs per year and should not exceed the direct costs of the parent grant, not including applicable Facilities and Administrative (F&A, indirect) costs. The earliest start date for this program is July/August 2024.
Budget FAQ
Applicants may request up to $250,000 in direct costs, not to exceed the direct costs of the parent grant, excluding F&A costs. In this case, you may request up to $130,000 in direct costs, because this is the current year direct cost budget for your grant.
Highly Resourced Institutions (as defined in the NOSI) can request support for one year only. Limited-Resourced Institutions (as defined in the NOSI) may request up to two years of funding.
The budget cap is $250,000 direct costs per year (potentially up to $500,000 total).
Subject to the availability of appropriations, funds will be distributed July/August 2024.
You are not limited in the number of trainees to add. Applicants may request up to $250,000 in direct costs, not to exceed the direct costs of the parent grant, excluding F&A costs. With regard to the application submission, please include each trainee’s biosketch and include in the Personal Statement information about the trainee's motivation to enter and remain in biomedical and behavioral research, and their short-term and long-term career goals.
Allowable costs for NOT-OD-24-001 are those allowed in the parent grant. Thus, although there is language in the NOSI suggesting a range of ways in which the funds can be used, the investigator should be aware that what is allowable in the parent grant dictates what is allowable in the supplement. If clarification is required, please consult with the IC Point of Contact.
Yes, supplement awards may provide support above the established parent NOFO budget limits. The application budget should not exceed $250,000 in direct costs per year and should not exceed the direct costs of the parent grant. In this case, the direct costs of the parent grant are $250,000, so the application budget should not go beyond that amount. If the direct costs of the parent grant were $125,000, then that application would be limited to a request of $125,000.
Administrative supplements are not program announcements with set-aside funds (PAS). As such the number of awards is contingent upon NIH appropriations.
Application
The proposed work for this initiative must be within the scope of the parent award and must be submitted using the following opportunity or its subsequent reissued equivalent: PA-20-272 - Administrative Supplements to Existing NIH Grants and Cooperative Agreements (Parent Admin Supp Clinical Trial Optional). Complete applications must be submitted by February 16, 2024 by 5:00 PM local time of applicant organization.
Applicants are strongly encouraged to consult with the Program Official for the parent grant or the contact listed in the institute/center scientific contact table linked above to confirm eligibility and to obtain technical assistance. Applicants are also strongly encouraged to notify the program contact at the Institute supporting the parent award that a request has been submitted in response to this NOSI in order to facilitate efficient processing of the request.
Application FAQ
All instructions in the SF424 (R&R) Application Guide and PA-20-272 must be followed, with the following additions:
- include “NOT-OD-24-001” (without quotation marks) in the Agency Routing Identifier field (box 4B) of the SF424 R&R form.
- Proposed Activities
- Senior/Key Person Profile (Expanded) form
- R&R Other Project Information form, in the "Other Attachments" field:
- DEIA Statement of the PD/PI
- a list of current and former trainees/mentees that the PD/PI directly mentored and their outcomes.
- a brief statement regarding the current amount of unobligated grant funds and expenditure plans
- If applying as an LRI, an "Institutional Commitment Letter" (titled as such) from the institution leadership
Please refer to the NOSI for more information and instructions.
The PD/PI requesting support submits the DEIA Statement.
A supplement can start at any time during the parent project period but must end by the last day of the parent project period. Most administrative supplements are for a one-year period, and it is expected that the funds will be expended in that time. If the grant has less than a year left on it, then the funds need to be spent in that shorter period.
The Specific Aims and Research Strategy must be submitted on the PHS 398 Research Plan form. Applicants are allowed one page for Specific Aims and up to three pages for the Research Strategy section. The Research Strategy section should include a summary or abstract of the funded parent award, as well as an explanation of how the additional funds allow the PD/PI to support mentees while expanding the research program within the scope of the parent award.
The list should include those whom the PD/PI has mentored over the previous 10 years.
The list only includes trainees/mentees that the PD/PI has directly trained/mentored and their outcomes. The mentorship structure may take many forms (e.g., dyadic, etc.) and describes “mentors and mentees as both providers and recipients of unique information and access to resources” (NASEM, 2019 and references therein).
List Senior/Key Personnel who are being added to this supplement, or for whom additional funds are being requested through this supplement; include a biographical sketch for each. No need to include every Key Personnel on the parent grant.
If you have references, they can be added in the “Bibliography and References Cited” section as stated in the SF424 (R&R) Application Guide.
Find the application package linked from the NOFO that is appropriate for the parent award. For instance, for research grants (e.g., R’s, DP’s), cooperative agreement equivalents, and endowment programs, P’s, use the “research” package. For institutional training (e.g., T’s), use the “training” package. More information is available at this link: https://grants.nih.gov/grants/administrative-supplements.htm.
It is not allowable to have duplicate or highly overlapping applications under review at the same time as per NOT-OD-18-197. If your submission to this administrative supplement is sufficiently distinct, then you may submit it.
No, only one request per supported PD/PI will be considered.
No. Each application must be sufficiently distinct from any other administrative supplement currently under consideration by the awarding NIH institute or center.
Note: Candidates for the diversity supplement (PA-21-071) may receive support from only one administrative supplement at a time but may be supported by more than one supplement during the development of their research careers.
Yes, funds can be used for other performance sites so long as the activities are within the scope of the parent award. Please follow the instructions in the Administrative Supplements to Existing NIH Grants and Cooperative Agreements (Parent Admin Supp Clinical Trial Optional).
All key personnel must obtain eRA Commons IDs. The Authorized Organization Representative/ Signing Official (AOR/SO) at your institution will be able to assist with assigning the eRA ID. NOT-OD-13-097 provides more information.
The staff of the NIH awarding component will evaluate requests for a supplement to determine its overall merit. The following general criteria will be used that define outstanding mentorship and eligibility for this supplement award:
- Proposed work is within the scope of the active award
- Strength of the PD/PI’s commitment to their trainees’ scientific and professional development and career progress within the biomedical research enterprise
- Strength of the PD/PI’s demonstrated commitment and contributions to enhancing diversity, equity, inclusion, and accessibility in the biomedical sciences.
- The extent to which the proposed activities will:
- have a broader impact on the training environment
- develop or enhance skills in working effectively with talented scientists from a wide variety of backgrounds and to promote inclusive, equitable, and accessible scientific biomedical research environments
- have positive outcomes and meaningful impact to enhance DEIA
- be completed within the proposed timeline
The following will be considered in making funding decisions consistent with applicable law:
- Scientific and technical merit of the proposed project
- Relevance of the proposed project to program priorities
- Diversity of
- Career Stage
- Geographic distribution
- HRIs and LRIs
- Availability of funds
Review criteria will assess both past contributions and future proposed activities for DEIA and mentorship. Past contributions are determined by:
- Strength of the PD/PI’s commitment to their trainees’ scientific and professional development and career progress within the biomedical research enterprise
- Strength of the PD/PI’s demonstrated commitment and contributions to enhancing diversity, equity, inclusion, and accessibility in the biomedical sciences
Future proposed activities are determined by the extent to which they will:
- have a broader impact on the training environment
- develop or enhance skills in working effectively with talented scientists from a wide variety of backgrounds and to promote inclusive, equitable, and accessible scientific biomedical research environments
- have positive outcomes and meaningful impact to enhance DEIA
- be completed within the proposed timeline
The progress report is not part of the application but rather part of the reporting requirements. The progress report and budget for the supplement must be included with, but clearly delineated from, the progress report and budget for the parent award. The progress report must include information about the activities supported by the supplement even if support for future years is not requested.
Funding decisions will be made in time for an earliest start date of July/August 2024. Administrative supplements are administratively reviewed by NIH staff and are not reviewed by scientific review groups. As such, there are no summary statements.

